Abstract
Primary CNS Lymphoma (PCNSL) with diffuse large B-cell lymphoma (DLBCL) histology occurring after organ transplantation (EBV+ PCNSL-PTLD) is characterized by extremely poor outcome, almost universal EBV-positivity and late presentation relative to systemic PTLD (Fink, AJT 2012; Evens, AJT 2013). However, as the incidence is low and biopsy material limited, characterization of its immunobiology is minimal. Furthermore, there is no comparative data with EBV+ DLBCL occurring as systemic PTLD (EBV+ syDLBCL-PTLD), or with EBV-ve DLBCL in the non-PTLD setting occurring either as PCNSL (EBV-ve PCSNL) or systemically (EBV-ve syDLBCL).
Here, we outline results of a detailed comparison of the genetic landscape and tumor microenvironment (TME) between PCSNL and systemic DLBCL (syDLBCL), stratified by EBV and PTLD status. A combination of targeted sequencing (involving NFk-B, immune response, cell cycle and epigenetic genes), CNV analysis, nanoString gene expression (for macrophage, immune checkpoint and effector molecules), and in-vitro assays was employed. 191 adult patients with DLBCL (13 EBV+ PCNSL-PTLD; 27 EBV-ve PCNSL; 11 EBV+ syDLBCL-PTLD; and 140 EBV-ve syDLBCL) were included, with 16 non-malignant lymph nodes as controls.
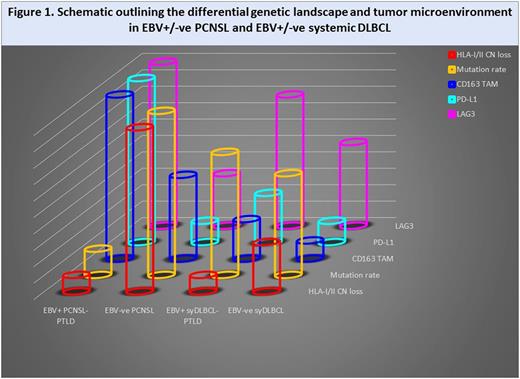
EBV+ PCNSL-PTLD was typically viral latency III. In EBV-ve PCNSL, in broad agreement with previous studies (Gonzalez-Aguilar, CCR 2012; Chapuy, Blood 2015; Fukumura, Acta Neuropath 2016; Nakamura, Neuropath Appl Neurobiol 2016), common nonsynonymous mutations in known cancer drivers were PIM1 (76%), MYD88 (72%), CD79B (68%), TBL1XR1 (48%), KMT2D (44%), TOX (28%), PRDM 1 (24%), EP300, CREBBP1 and CIITA (all 20%). Interestingly, the mutation rate in EBV-ve PCNSL was considerably higher than in EBV+ PCNSL-PTLD, and was also higher than EBV-ve syDLBCL and EBV+ syDLBCL-PTLD.
In line with previous reports, there was high occurrence of CN loss at the HLA-class I/II loci (Riemersma, Blood 2000) in EBV-ve PCNSL. However, this was relatively infrequent in EBV+ PCNSL-PTLD and EBV+ syDLBCL-PTLD, and intermediate in EBV-ve syDLBCL. To investigate the TME gene expression profile, a selection of immune effector and checkpoint genes were quantified in combined PCNSL cases and compared to syDLBCL i.e. irrespective of viral status. CD4 and CD8 levels were similar in PCNSL vs. sysDLBCL, whereas the NK cell marker CD56 was 9-fold higher in PCNSL (p<0.0001). A key feature was that the immune activation marker CD137 was 2-fold higher in syDLBCL than PCNSL (p<0.0001), indicative of reduced immune effector function in PCNSL. Consistent with this, there was a 6-fold increase in the M2 immunosuppressive tumor-associated macrophage marker CD163 in PCNSL vs. syDLBCL (p<0.0001). Furthermore, CD4 and CD8 correlated with CD163, suggesting an adaptive immune response in which the malignant B-cell up-regulates M2 macrophages to evade immune surveillance. To test this, healthy monocytes were co-incubated with a PCNSL cell-line. This resulted in the elevated expression of CD163+, PD-L1+ and PD-L2+ M2 polarized monocyte/macrophages in PCNSL relative to a syDLBCL control cell-line (all ≥7-fold, p<0.0001), confirming the differential impact of PCNSL to upregulate immunosuppressive monocyte/macrophages.
Next, differences between the TME in EBV-ve PCNSL and EBV+ PCNSL-PTLD were examined. PD-1 levels were similar. However, consistent with our previous findings in EBV+/-ve Hodgkin Lymphoma, levels of CD163 (Jones, CCR 2012) and LAG3 (Gandhi, Blood 2006) genes were both 4-fold higher in EBV+ PCNSL-PTLD biopsies than EBV-ve PCNSL (both p≤0.005). Furthermore, both PD-L1 and PD-L2 were 7-fold and 3-fold higher in EBV+ PCNSL-PTLD than EBV-ve PCNSL respectively (both p<0.005). These data implicate EBV as a major determinant of the TME within the CNS.
Combined, these results indicate that 'immune privilege' occurring in the context of lymphoma within the CNS is more accurately described as an adaptive immune response in which the malignant B-cell actively utilizes a variety of mechanisms to evade immune surveillance (Fig 1). For EBV+ PCNSL-PTLD this involves up-regulation of PD-L1+/PD-L2+ M2 monocyte/macrophages and LAG3, whereas with EBV-ve PCNSL the emphasis is on genetically mediated immune evasion including loss of HLA-I/II loci. The findings will help guide the rational design of novel immunotherapeutic strategies for EBV+ PCNSL-PTLD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.